Refractometry: A Valuable PAT Tool for Pharmaceuticals and Biotechnology
Evolution, from batch process to continuous production
Batch processes are the most commonly used production method in the pharmaceutical industry, as well as in related sectors such as biotechnology and cosmetics. Despite this, the FDA, among others, has been supporting and promoting the transition to continuous production processes for around a decade. Continuous processes provide numerous advantages, including permanent quality control, shorter turnaround time, higher efficiency and reduction of human errors.
Process Analyser Technology (in short: PAT) is inextricably linked to continuous processes, but it is also indispensable in batch processes. Inline and online analysers provide continuous information about the reactions and process steps. This provides a contribution to process and product knowledge that should not be underestimated. Ultimately, this leads to better upscaling and more robust processes in a shorter time.
Inline refractometry
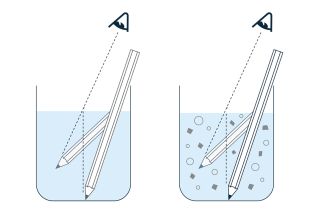
PAT is useful in both batch and continuous processes. Inline process monitoring in batch processes guarantees a "Batch-to-Batch-Consistency". Refractometry, or refractive index measurement, is a PAT technique that is simple to implement, but that makes a major contribution to “Process understanding”. This measurement technique determines the total volume of dissolved substances, i.e. the total concentration, continuously and without delay (real time). In other words, it is the ideal tool for permanent monitoring of the composition of the product.
Analysers that are installed directly in the process must, of course, meet specific requirements for the pharmaceutical and biotechnology industry. Hygienic design, FDA-approved materials and resistance to CIP/SIP are only a few examples.
VAISALA K-Patents inline refractometers comply with all essential requirements.
Scaling up - Make it easy on yourself
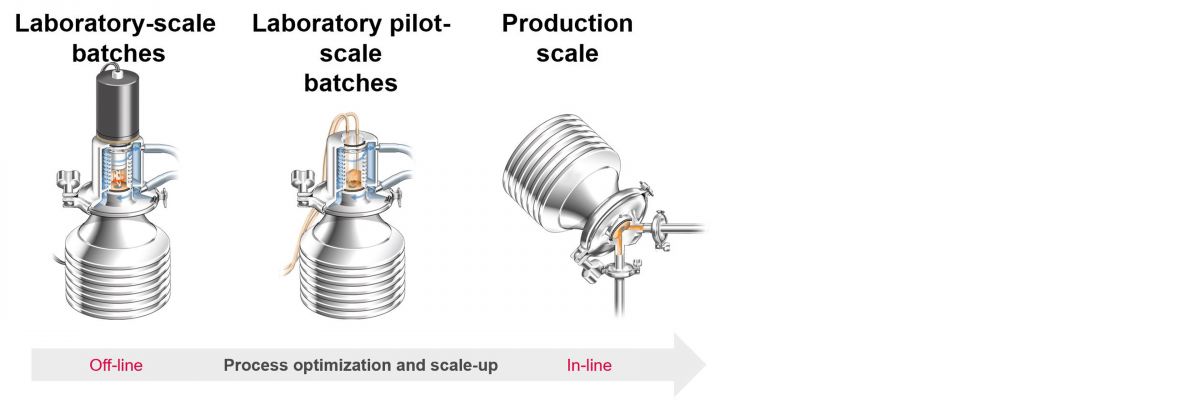
One of the major challenges in "scaling up" projects is the use of different analysis systems during the different steps. Laboratory analyses are simply not the same as inline measurements. An inline refractometer of the same type can be used for small volumes in the R&D environment, in the pilot installation and in final mass production. This eliminates many errors and facilitates the scaling up process.
No sampling required
A refractometer is installed directly in the process pipe (from 4 mm diameter) or reactor. The measurement is actually performed in the process at the prevailing process conditions. No sampling or sample preparation is required, nor any filtration or dilution. Moreover, refractometry is the only technique for determining the concentration of dissolved substances that is not disturbed by suspended particles or air bubbles!
Some examples
Inline refractometers are used in numerous pharmaceutical applications, including biopharma, chempharma and medical technology. We provide a brief overview of the possibilities. You are sure to find inspiration!
-

BIOPHARMA
- Evaporation
- Fermentation (sugars, antibiotics, etc.)
- Extraction (plant extracts or other natural products)
- Filtration (virus-rich fraction in vaccines, UF blood plasma, proteins)
- Mixing, diluting and blending (media and buffer preparation, quality control, reducing mixing times, etc.)
- Final product quality control
-

CHEMPHARMA
- Dissolution
- Synthesis (conversion rate, endpoint determination in API production)
- Formulation
- Filtration (filter cake)
- Solvent recovery
- Crystallisation
- Mixing, diluting and blending (media and buffer preparation, quality control, reducing mixing times, etc.)
- Final product quality control
-

MEDICAL TECHNOLOGY
- Coagulation
- Chemical baths (dip baths, passivation baths for surface treatment, etc.)
- Final product quality control
Implementation as it should be
VAISALA K-Patents refractometers are available with the required documentation for IQ/OQ and PQ. The verification standards are NIST-traceable. The security of the electronic data has also been given the required attention and each activity is stored with a date/time stamp. This guarantees the integrity of the results.
Due to the unique construction of the "core optics", this type of refractometer is very stable and therefore requires little or no maintenance. It has no moving parts and the quality of the measurements is constantly monitored by internal algorithms.
Conclusion
When implementing PAT in pharmaceutical or biotechnology processes, an inline refractometer is a good choice as it gives you constant information about the process. This type of analyser can also be used in many different processes, from mixing and dilution, fermentation and solvent swaps to quality control during the process and on the finished product.