PIC/S standardisation for hospital pharmacies
For the sake of protecting public health, medicines must be of high quality, safe and effective. This requires them to be consistently prepared to appropriate quality standards. The PIC/S guide to good practice in preparing medicines in care facilities was created for this reason.
From 2026, every hospital in Belgium that manufactures its own medication will have to comply with PIC/S standards. These guidelines ensure that medicines are produced in a safer and better-controlled manner, providing better protection for both the pharmacist and the products.
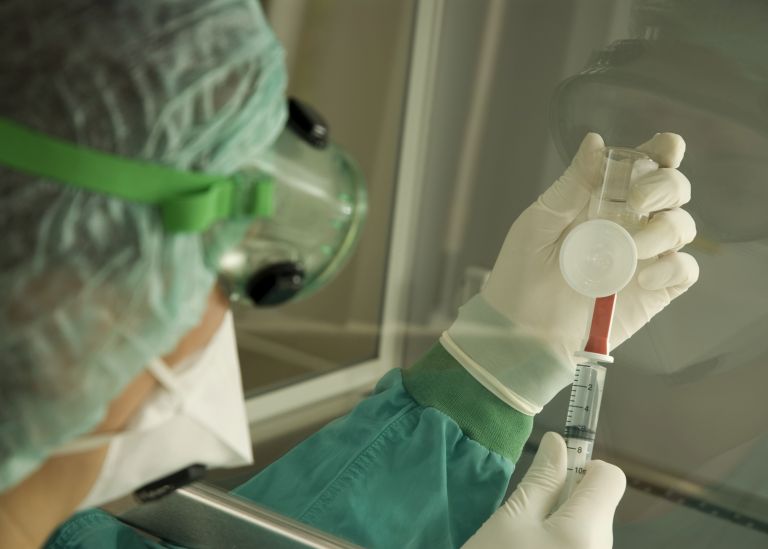
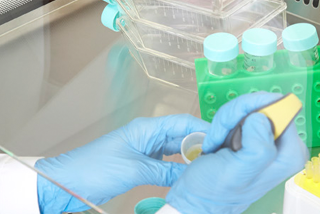
When it comes to sterile preparations, it is very important that appropriate precautions are taken to avoid contamination of the product through operator contact. They must therefore be prepared in a clean room, which is a defined area where contamination is reduced using specific filter systems.
Aseptically prepared products should also be processed in an LAF cabinet or isolator. Isolators in particular will involve a major adjustment in the daily operations of hospital pharmacies. Isolators, or so-called glove boxes, provide complete separation between the operator and the product and provide the very highest level of safety. However, they should be decontaminated after each use. Faster's GloveFAST Isolators have an easily removable stainless-steel worktop and inflatable gaskets that allow for safer and faster decontamination.
Services that are outsourced to a third party, such as the maintenance of isolators and laminar flow cabinets, must meet specific quality requirements that are set out in a "Service Level Agreement". Elscolab can also assist with this.
More information?