How do you choose the right electrode for your specific application?
For most of us, pH measurement has become so common that we hardly pay attention to it. However, the choice of the right pH electrode is very important. The correct electrode used in the right application reduces costs on maintenance and calibration. On top of that, it increases reliability which has a positive influence on process safety, product quality or efficiency. But, how do you choose the right electrode for your application?
To make the right choice, it is interesting to know how an electrode looks like and what the function of its components is. With that knowledge in mind, choosing the most appropriate electrode is much easier.
Construction of a pH electrode
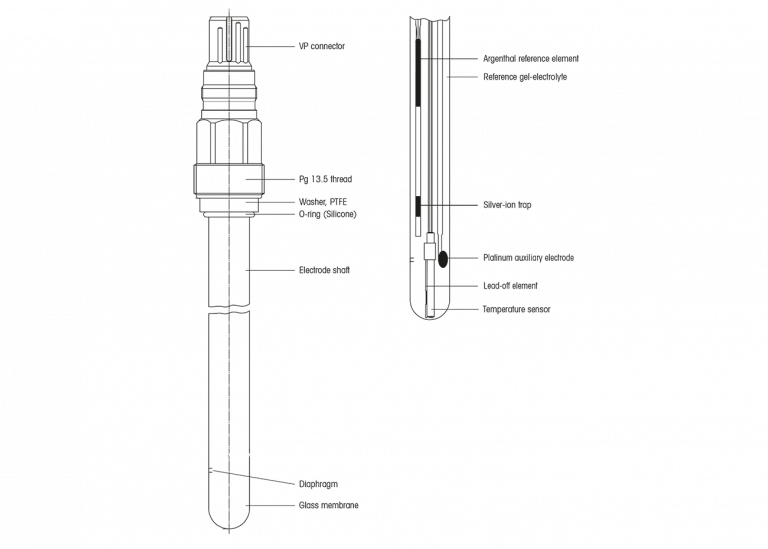
The pH electrode we commonly use today is actually a combined electrode. This means that it consists of the actual measuring electrode and a reference electrode. Often with a built-in temperature sensor. In most cases the measuring electrode is made of glass, but it can also consist of other material like enamel or a microchip (ISFET). Make no mistake, an ISFET electrode is also covered with a fine glass layer. There are also other types such as optical pH probes, but we shall not be considering those here.
Inside the glass electrode, in the bulb, you find a liquid. This is the inner buffer solution, a pH buffer with a known and fixed pH value. In theory this value can be anything but in the majority of cases this is a buffer with a pH value of about 7.00. In the inner buffer a lead-off system is immersed which is connected to the signal transmitter through a connector. Enamel and ISFET electrodes do not have inner buffer solutions. The potential is directly measured by the transmitter.
In the vicinity of the measuring electrode you will find the diaphragm(s). It is an opening which connects the measured liquid to the reference electrode. Mostly this diaphragm is a porous material, made out of ceramic, wood, PTFE, … but it can also be an open junction. Specifically for lab electrodes it can also be a sleeve diaphragm. On the inner side of the diaphragm is a liquid called reference electrolyte. It is a concentrated salt solution (normally potassium chloride, KCl 3 molar ion activity). In this electrolyte the actual reference electrode is immersed, together with its lead-off wire, in a silver chloride environment, the Ag/AgCl reference system. The reference electrode is also connected to the signal transmitter. The transmitter measures the difference in potential between glass- and the reference electrode. This difference in potential is electrically transferred and converted to a pH value.
This brief description of a classical pH electrode is sufficient for the purposes of this article. In reality, a pH electrode is much more complicated.
To be more complete we need to mention that a pH electrode may contain a temperature sensor for compensation purposes.
Almost all conventional pH electrodes are constructed as discussed. Where are the subtle differences between various types?
The glass
The pH sensitive glass is the actual detection element or sensitive part of the sensor. Its composition is one of the manufacturer’s best kept secrets and is therefore never disclosed. There are many different types of glass, suitable for one or more typical applications. An example is the special glass composition for electrodes used in the pharmaceutical or biotech industry, where electrodes are exposed to CIP/SIP cycles. In the food industry, glass is often not allowed in processes or process environment. Therefore they prefer, in most cases, an ISFET electrode. However, take into account also an ISFET electrode has a glass layer. Enamel electrodes can be a good alternative solution in this situation.
In applications where electrodes need to be sterilised or autoclaved, it is best to opt for a glass type with better zero stability. This means that such electrodes can be sterilised and/or autoclaved more often than standard glass electrodes. For use in very cold solutions (at 0°C or below) as well as for media containing fluorides, special glass types exist.
The diaphragm
The diaphragm is, as mentioned here above, the connection between the medium to be measured and the reference electrode. Mostly the diaphragm is porous. The pore size and the number of diaphragms depends, among other things, on the type of reference electrolyte. Electrodes with polymer electrolyte don’t have a porous diaphragm, but an open junction. In general, these types of electrodes are used in media containing suspended solids or in dirty solutions, where diaphragm potentials are expected. Contrary to what people commonly think, the diaphragm is the main source of measuring errors or malfunctioning of the electrode, mainly due to contamination. Therefore it is of the utmost importance to keep the diaphragm clean during measurement by making sure there is a constant outflow of reference electrolyte through the diaphragm. This can be achieved by internal or external pressure on the electrolyte.

The reference electrolyte
The electrolyte is almost always based on potassium chloride, KCl, with an activity of 3 moles per liter (3M). Pure 3M KCl is preferred mostly. In some cases it may be useful to choose an electrolyte which contains additives to the KCl, which improve the electrode for use in cold environment or organic media. Electrodes with liquid electrolyte should always be used with a pressure on the electrolyte which is higher than the process pressure to guarantee an outflow of electrolyte in order to keep the diaphragm clean. Nowadays more and more ‘solid’ electrolytes are used: gel or polymer. Their advantage is that the installation in an industrial process is easier and requires less maintenance. However, stability, calibration intervals and life time are often less than for electrodes with liquid electrolyte under pressure.
The solution ground
More and more industrial electrodes are provided with a built in solution ground. For classic analog electrodes it serves to prevent electrical interferences. If the solution ground is made of platinum, it can be used as an ORP (redox) electrode, simultaneously to the pH measurement. When using an appropriate transmitter you can measure the reference impedance of an electrode with solution ground. This way, in some cases, contamination can be detected.
Digital or analog
Different suppliers now offer digital and/or intelligent pH electrodes. The main advantage is that this reduces problems related to electrical interference and installation. Think in particular of problems which occur due to humidity and corrosion on cables and connectors. In addition, the built-in intelligence provides extra tools to monitor the status and expected life time of the electrode. In digital electrodes the analog mV signal is converted in the head of the electrode to a digital signal. This digitalisation creates the possibility to check and even calibrate your electrode in the lab or work place, if necessary with the assistance of a PC or smartphone/tablet. The digitalisation of a pH electrode has no influence on the actual measurement. However, digital and intelligent electrodes are the future.
Choosing a pH electrode
From the above, it can be concluded that choosing the right pH electrode is not always easy. Therefore, it is a good idea to discuss your choice with your supplier. To enable him/her to propose the most suitable sensor, it is important to provide all possible information about your application. This concerns the pH range, the pressure and temperature and even the chemical composition of the medium and how the sensor has to be mounted in the process.
More information?