Chemistry - Dilution
Most chemicals are supplied in concentrated solution or even in solid form. They must be diluted before use in a production process. Usually with water, sometimes also with other solvents. A concentration measurement is the key instrument when making a dilution. Ultimately, you want the right concentration!
Diluting or mixing: the concentration gives the correct result.
The mixing or dilution process can be monitored in the tank itself or in a circulation line. You can choose from different analysis techniques depending on the product.
Refractometry or refractive index
In many cases this is the best choice. This analysis technique is not affected by gas bubbles or particles and requires little or no maintenance.
Conductance or conductivity measurement
These types of sensors are ideal for concentration measurements of some common salts, acids and bases such as NaCl, hydrochloric acid, sulphuric acid, NaOH, etc.
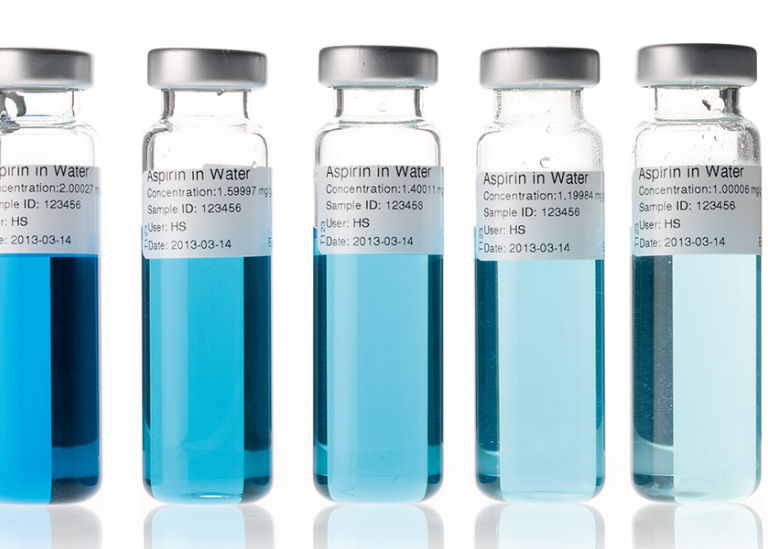
Photometry/colour measurement
The colour, or colour intensity, of a product is often proportional to its concentration. A photometer is significantly more sensitive than the human eye and therefore measures the concentration of your product very accurately.
pH measurement
In addition to pH measurement, consider redox or ORP measurement. These measurements don’t really determine the concentration, but a chemical property of the solution. In this case, the acidity or the oxidation power (redox potential). If that property is important for the process or product quality, it’s best to measure it directly.
Turbidity measurement
Sometimes it is not about a real solution, but about a suspension (undissolved particles in a liquid). Then turbidity or cloudiness is the best measurement technique.
More information?